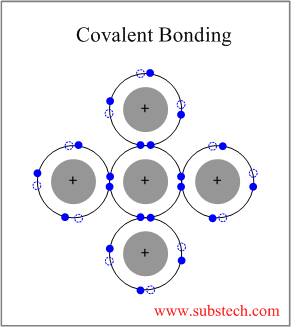
They are either ionic in character involving a transfer of bonding electrons from electropositive atoms to electronegative atoms or they are covalent in character involving orbital sharing of electrons between the constituent atoms or ions.
Do ceramics contain ionic bonds.
Compounds that are either mostly ionic or mostly covalent have higher melting points than compounds in which neither kind of bonding predominates.
These chemical bonds are of two types.
High hardness high compressive strength and chemical inertness.
The bonding of atoms together is much stronger in covalent and ionic bonding than in metallic.
For metals the chemical bond is called the metallic bond.
So if a row of atoms attempts to slide past the next row of atoms this would move positive ions towards positive ions and negative ions towards negative ions.
Electronegativity is the capability of the nucleus in an atom to attract and retain all the electrons within the atom itself and depends on the number of electrons and the distance of the electrons in the outer shells from the nucleus.
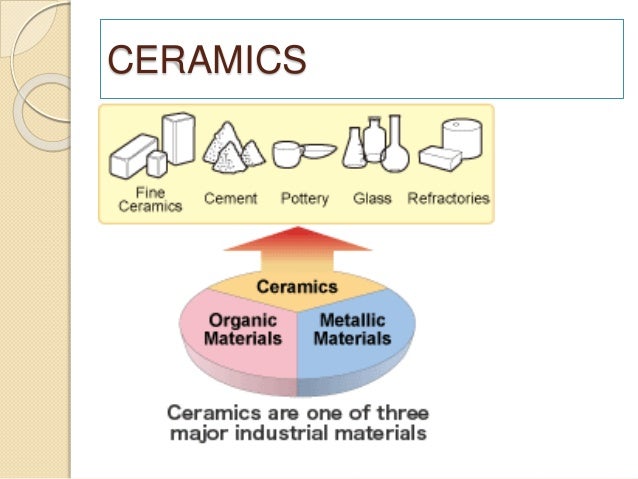
Ceramic materials are usually ionic or covalent bonded materials and can be crystalline or amorphous.
The ions pack into a regular arrangement.
The overall properties of these materials depend on the dominant bonding mechanism.
A material held together by either type of bond will tend to fracture before any plastic deformation takes place which results in poor toughness in these materials.
Two types of bonds are found in ceramics.
This causes bonding between atoms.
The two most common chemical bonds for ceramic materials are covalent and ionic.
This is due to the fact that in a ceramic we have predominately ionic bonding which results in positive and negative ions alternating.
This is why ceramics generally have the following properties.
You can recognize ionic compounds because they consist of a metal bonded to a nonmetal.
Ionic bonding is found in many ceramic structures such as nacl mgo and al2o3.
The bonding of atoms together is much stronger in covalent and ionic bonding than in metallic.
The ionic bond occurs between a metal and a nonmetal in other words two elements with very different electronegativity.
That is why generally speaking metals are ductile and ceramics are brittle.
Underlying many of the properties found in ceramics are the strong primary bonds that hold the atoms together and form the ceramic material.
Ionic bonds form between two atoms that have different electronegativity values because the ability to attract electrons is so different between the atoms it s like one atom donates its electron to the other atom in the chemical bond.
Atoms have unlike electrical charges making them ions which create an electrostatic attraction between atoms.
The two most common chemical bonds for ceramic materials are covalent and ionic.
The atoms in ceramic materials are held together by a chemical bond.